| CAS
NO. |
9067-32-7 |
|
| EINECS
NO. |
232-678-0 |
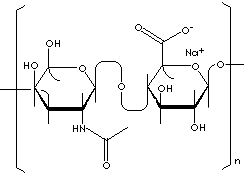
| FORMULA |
(C14H20NO11Na)n |
| MOL
WT. |
800,000
- 1,600,000 |
|
H.S.
CODE
|
|
| SMILES |
|
|
TOXICITY
|
Oral
rat LD50: 800mg/kg
|
| SYNONYMS |
Hyalgan;
|
| Hyaluronic
acid, sodium salt; Healon; Hyalurone sodium; |
|
CLASSIFICATION
|
|
|
PHYSICAL
AND CHEMICAL PROPERTIES
|
| PHYSICAL
STATE |
White
powder |
| MELTING
POINT |
|
| BOILING
POINT |
|
| SPECIFIC
GRAVITY |
soluble |
| SOLUBILITY
IN WATER |
|
| pH |
5.0
- 8.5 |
| VAPOR
DENSITY |
|
|
AUTOIGNITION
|
|
|
NFPA
RATINGS
|
|
|
REFRACTIVE
INDEX
|
|
| FLASH
POINT |
|
| STABILITY |
Stable
under ordinary condiions |
|
APPLICATIONS
|
| Hyaluronic
acid, a kind of mucopolysaccharides (also known as glycosaminoglycans),
is an integral part of the gellike substance found in
animal connective tissue, synovial fluid of joints and
the vitreous humor of the eyes. It contains amino-sugar
unit and uronic acid. It has the physiological function
of absorbing and retaining water to form jelly matrix
for the maintaining cell structure and protecting cell
against bacterial infections. Commercially, various
HA products are available for ophthalmology, arthritis
and odontology. They are widely used in cosmetics and
dermatologists for antiaging and moisturizing. |
| SALES
SPECIFICATION |
|
SOLID
|
|
APPEARANCE
|
White
solid |
| CONTENT |
85.0%
min
|
| NITROGEN |
2.8
- 4.0% |
| GLUCURONIC
ACID |
40.0
± 3.0% |
| ARSENIC |
2ppm |
| HEAVY
METALS |
20ppm
|
| LOSS
ON DRYING |
15.0%
max |
|
INJECTION
|
|
DESCTIPTION
|
Sodium
hyaluronate for ophthalmic surgery, pre-filled syringe |
| COMPOSITION |
10mg/ml |
| INJECTION
VOLUME |
-
Cataract: 0.2~04 ml
- Glaucoma: 0.3~0.5 ml
- Keratotransplantation:
0.2~0.6 ml
- Trauma: 0.1~0.6 ml
|
|
UNIT
SIZE
|
0.4ml,
0.55ml, 0.6ml, 0.85ml, 1.0ml in pre-filled syringe/pack
|
| STORAGE
CONDITION |
light-resistant
hermetic container at 2 C in the refrigerator |
| SHELF
LIFE |
36months
|
| TRANSPORTATION |
| PACKING |
|
| HAZARD
CLASS |
|
| UN
NO. |
|
| OTHER
INFORMATION |
| |
